RNA-based approach identified for treatment of premature aging and associated diseases

KAUST scientists are researching RNA-based approaches to treat progeria, an early aging disease that affects children and adolescents.
Scientists at King Abdullah University of Science and Technology (KAUST), in collaboration with a team at the Salk Institute and Alto Labs, have discovered a specific class of RNA that is responsible for maintaining the genetic integrity and function of cells and tissues in human genomes is compromised in people with progeria, an early aging disease.
Progeroid syndromes, which include Hutchinson-Gilford Progeria syndrome and Werner syndrome, cause accelerated aging in children and adolescents. Patients not only develop striking physical appearances, but also symptoms and diseases typically associated with older age, such as heart disease, cataracts, type 2 diabetes, osteoporosis and cancer. There are currently no effective treatments for progeroid syndromes.
The scientists devised an antisense RNA strategy to block the aberrant function of L1 RNA and, in this way, successfully reversed the disease in mice and patient derived primary cells. Their research, published in Science Translational Medicine, focuses on a type of RNA known as LINE-1 (long interspersed nuclear element-1; L1).
"Targeting LINE-1 RNA may be an effective way to treat progeroid syndromes, as well as other age-related diseases that have been connected to LINE-1, including neurodegenerative and metabolic disorders, cancers and certain aspects of tissue regeneration," said KAUST team lead Dr. Valerio Orlando, professor of bioscience and head of the Environmental Epigenetics Program. "Eventually, we think that this approach may lead to treatments to help extend human health span."
"These findings provide new insight into progeroid syndromes and how to treat them, while also highlighting the importance of LINE-1 RNA in normal aging," added co-author Juan Carlos Izpisua Belmonte, a professor in Salk's Gene Expression Laboratory and holder of the Roger Guillemin Chair and Director of Altos Labs San Diego Institute of Science.
Orlando, Izpisua and colleagues knew that one of the molecular signatures of both normal aging and progeroid syndromes is the altered overall organization of DNA. When DNA is packaged differently into the nuclei of cells, it changes which genes are accessible for the cell to use, and can therefore drastically change a cell's behavior and function.
The human genome contains tens of thousands of LINE-1 elements — ancestors of viral genomes, a few of them still capable of propagating and moving around the genome via an RNA intermediate. The function of these elements is poorly understood, but they change and multiply with age, as well as in diseases including neurodegenerative, cancer and cardiovascular disease. The researchers wondered whether they also changed in progeroid syndromes.
"The repeat sequences like LINE-1 make up a large percentage of our genomes (17-20% of total DNA content), but not much attention is paid to the effects of LINE-1 RNA that increase with age in the nuclei," said Francesco Della Valle, KAUST research scientist and co-first author with Pradeep Reddy, a Salk staff scientist and principal scientist at Altos Labs.
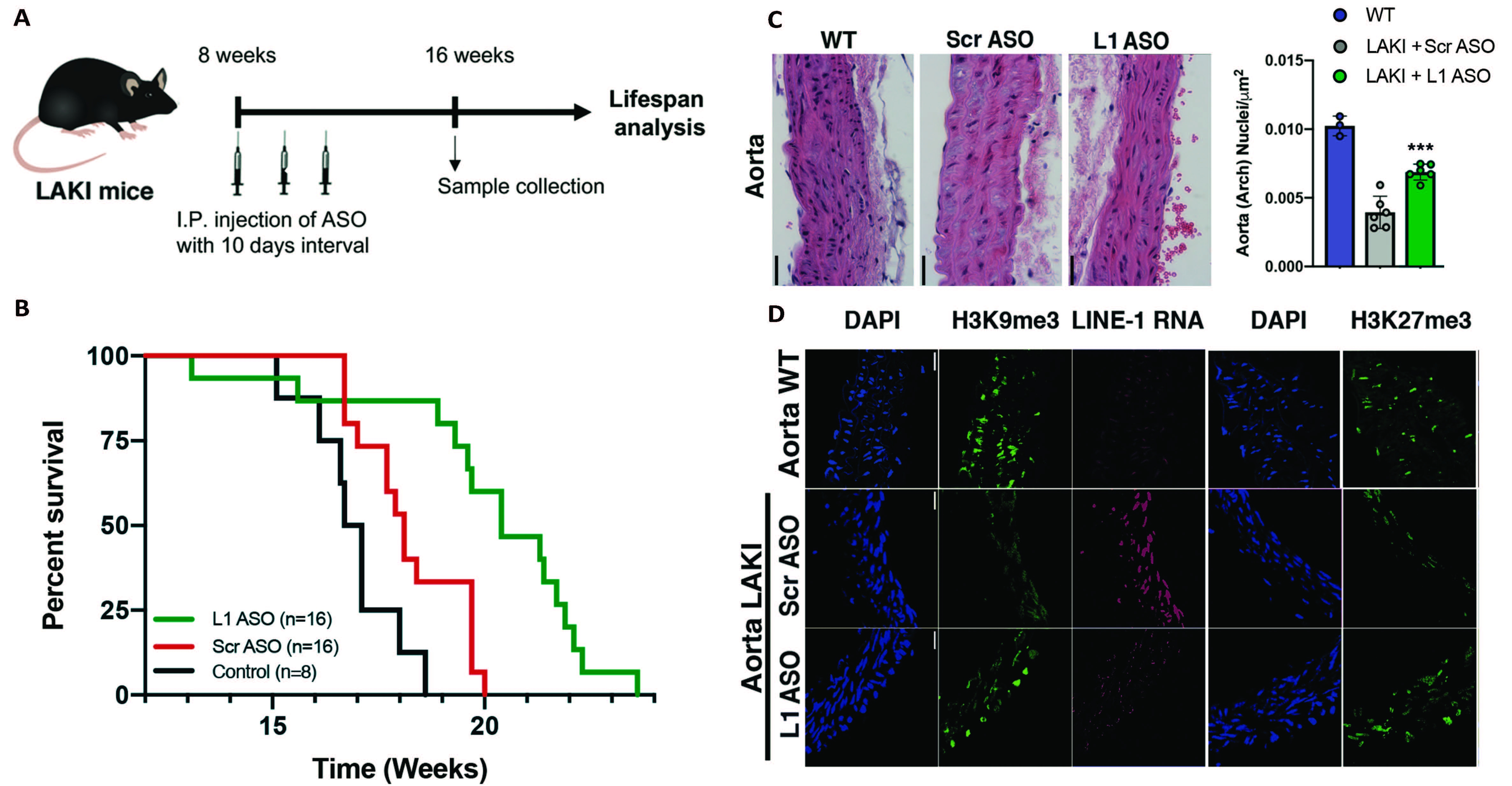
A) schematic representation of LAKI mice (Progeria mouse model) treatment with LINE-1 Antisense Oligonucleotides (L1 ASO) or scramble non-targeting ASO (Scr ASO). B) Lifespan analysis of LAKI mice treated with L1 ASO compared to groups of control. C) Histological analysis of nuclei density in the Aorta and the Skin of healthy (WT) or LAKI mice treated with L1 ASO or Scr ASO. D) Immunohistological analysis of heterochromatin associated histone mark H3K9me3 in the Aorta and the skin of healthy (WT) or LAKI mice treated with L1 ASO or Scr ASO.
The researchers studied cells derived from patients with progeroid syndromes and found that they had four to seven times more LINE-1 RNA than cells from healthy individuals. Moreover, they showed that the accumulation of LINE-1 RNA came before the major structural changes to nuclear organization and genetic program deviations that were already associated with progeria.
The team then developed molecules that could specifically bind to LINE-1 RNA, preventing the RNA from accumulating and impacting the cells. This kind of treatment reversed the molecular signs of progeria in isolated cells and extended the life span of mice with genetic mutations that typically cause premature aging. In both cases, the expression of genes associated with cell proliferation and tissue specific program increased after treatment, while the expression of genes associated with aging, inflammation and DNA damage decreased.
The researchers are planning future studies to improve the efficacy and tissue specificity of antisense L1 RNA targeting in vivo and also understand what causes the accumulation of LINE-1 RNA and how it can be prevented in human patients. A patent application for the current work targeting the LINE-1 RNA using antisense oligos has been filed.
The work was supported in part by KAUST (BAS/1/1037/01-01), the KAUST Competitive Research Grant Program, KAUST Smart Health Initiative, the Moxie Foundation and Universidad Católica San Antonio de Murcia.
Publication link:
LINE-1 RNA causes heterochromatin erosion and is a target for amelioration of senescent phenotypes in progeroid syndromes
Francesco Della Valle1†, Pradeep Reddy2,3†, Mako Yamamoto2,3, Peng Liu1,
Alfonso Saera4, Dalila Bensaddek5, Huoming Zhang5, Javier Prieto Martinez2, Leila Abassi1,
Mirko Celii1, Alejandro Ocampo2, Estrella Nuñez Delicado6, Arianna Mangiavacchi1,
Riccardo Aiese Cigliano4, Concepcion Rodriguez Esteban2,3, Steve Horvath3,
Juan Carlos Izpisua Belmonte2,3*, Valerio Orlando1*