Unique structure of chiral gold nanowires discovered by KAUST researchers
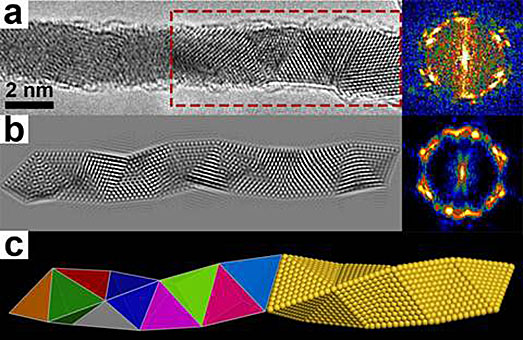
The gold nanowires produced by Prof. Yu Han’s research team take on a Boerdijk-Coxeter-Bernal (BCB) structure in these images, with a) and b) showing experimental and simulated high-resolution transmission electron microscopy (HRTEM) images of a single BCB-structured gold nanowire, respectively. C) shows topological (L) and atomic (R) models of a BCB tetrahelix.<br> Image credit: Yihan Zhu et al. J. Am. Chem. Soc. 2014, 136, 12746-12752.<a href="http://pubs.acs.org/doi/abs/10.1021/ja506554j">http://pubs.acs.org/doi/abs/10.1021/ja506554j</a>.
Yu Han, Associate Professor of Chemical Science, Yihan Zhu, a research scientist in KAUST’s Advanced Membranes and Porous Materials Research Center (AMPM), and a team from Nanyang Technological University in Singapore have made a breakthrough discovery in developing chiral metallic nanowires.
Their research was recently published in the Journal of the American Chemical Society (JACS; DOI: 10.1021/ja506554j), and was selected by the journal’s editor for JACS Spotlights.
Forming a BCB Helix
Chirality is an important property of molecules whereby the molecules are composed of the same atoms but are non-superimposable mirror images of each other. The chiral nanowires the team produced are formed by the close packing of gold atoms and form a close example of a Boerdijk-Coxeter-Bernal (BCB) helix, also known as a tetrahelix. The atomic packing mode of the gold nanowires is different from all other known examples in published scientific literature.
“The assembly of bulk chiral crystals has a long history, but producing chiral nanostructures is still an emerging field,” explained Prof. Han. “Chiral crystal formation from highly symmetric atoms is very important, as chiral crystals – and particularly metallic ones – can be used in many applications, including chemical separation, sensing and catalysis. However, although much is known about producing chirality in packed organic molecules, less is understood about chirality in metallic nanowires.”
Synthesizing Nanowires
The researchers synthesized the thinnest possible gold nanowires they could produce using a seed-mediated substrate growth method. The gold nanowires were grown on specially designed liquid/substrate interfaces, and reached a minimum diameter of 3 nanometers.
“At this lowest limit, the gold nanowires became chiral with the BCB-type structure,” said Prof. Han. “Interestingly, the regular BCB helical structure exists only with these ultra-thin gold nanowires. As the nanowires grow thicker, the unique helices no longer form.”
Importance of KAUST Facilities
High-resolution transmission electron microscopy (HRTEM) at KAUST was used to reveal the structure of the gold nanowires. “This was one of the most important tools we used to explore and eventually identify the delicate nanostructure. Without it, the research would have been impossible,” noted Dr. Zhu.
“An essential point we learned from the study is that to build a nanomaterial from the bottom up using chemosynthesis, we need to create an environment such that the target structure is the naturally occurring product,” he continued. “This is made possible by properly controlling the synthesis conditions.”
Wet-Chemistry Synthesis: Essential for the Future
The researchers hope the power of wet-chemistry synthesis has been shown through their study. Using this important approach, it is possible to build various nanostructures with specific properties from the bottom up, and it even enables the control of the atomic packing mode in the structures, they noted.
“We believe the understandings from our study are critical in the further exploration of chiral nanowires,” Prof. Han stated. “We have shown that it is possible to fabricate chiral metallic nanostructures from highly symmetric atoms, and the diversity of possible structures at the nanoscale has yet to be fully realized. Unusual atomic packing, like we showed in our study, is of great fundamental significance and has many important practical applications.”
- by Caitlin Clark, KAUST News