Materials Beyond: KAUST scientists put solid state materials design myth to rest
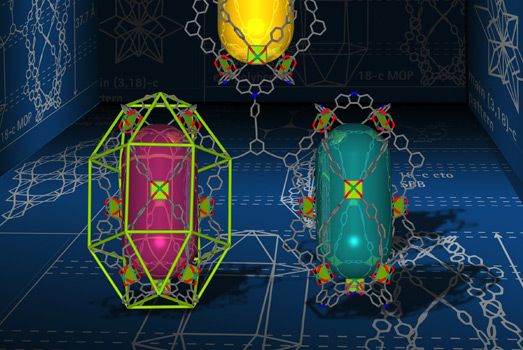
Prof. Mohamed Eddaoudi's Functional Materials Design, Discovery & Development group (FMD3) discovered a special minimal edge transitive net with high connectivity, pictured here. The image illustrates its use as a blueprint for the design and construction of metal organic frameworks (MOFs). The (3,18)-connected blueprint and the supermolecular building block (SBB) approach enabled the deliberate design and synthesis of a novel MOF. <br> Image credit: Prof. Mohamed Eddaoudi and Dr. Vincent Guillerm.
"Solid-state materials that have been designed and constructed in a made-to-order manner may provide potential solutions to many present-day challenges in energy and environmental sustainability," explains Dr. Mohamed Eddaoudi, KAUST Professor of Chemical Science and Associate Director of the University's Advanced Membranes and Porous Materials Research Center. "However, it is very difficult to design these materials, as their assembly is done in a one-step synthesis."
Prof. Eddaoudi's Functional Materials Design, Discovery and Development (FMD3) research group concentrates on developing strategies to permit the design of solid-state materials and the control of their functionality. In particular, the researchers focus on introducing certain specific properties and functionalities at the design stage prior to assembly in pre-selected molecular building blocks (MBBs).
The group research concentrates on metal-organic frameworks (MOFs), a promising class of modular solid-state materials, because MOFs can be constructed by the assembly of predefined MBBs approach.
"It is possible to mutually control the porous structure of MOFs and their composition and functionality," says Dr. Vincent Guillerm, a post-doctoral fellow in Prof. Eddaoudi's FMD3 group. "In our group, we look for novel highly connected MBBs and their transposition into nets with high connectivity."
"Targeting high connectivity MBBs will reduce the number of possible outcome nets and will therefore be of a great help on the road to the rational design of porous materials," notes Prof. Eddaoudi.
In a paper recently published in Nature Chemistry (DOI: 10.1038/NCHEM.1982), the group discovered and isolated highly connected polynuclear clusters which are used as MBBs in the synthesis of MOFs. In particular, they discovered and formed a new rare earth (RE) nonanuclear carboxylate-based cluster ([RE]9(μ-OH)8(μ2-OH)3(O2C-)18],(RE=Y, Tb, Er, Eu)), which was then used as an 18-connected MBB to form a gea-MOF (gea-MOF-1) based on an unprecedented (3,18)-connected net. "gea" stands for "Guillerm, Eddaoudi net A."
"We then utilized the gea net as a blueprint to design and assemble another MOF, called gea-MOF-2," explains Dr. Guillerm. "In gea-MOF-2, the 18-connected RE clusters are replaced by metal-organic polyhedra which have been peripherally functionalized to have the same connectivity as the RE clusters. The metal-organic polyhedra act as supermolecular building blocks, or SBBs, when they form gea-MOF-2."
"This required the challenging synthesis of a very specific, asymmetric, trefoil organic ligand," notes Dr. Łukasz Weseliński, post-doctoral fellow in the FMD3 group and second author of the paper.
"The discovery of a (3,18)-connected MOF followed by the deliberate transposition of its topology to a predesigned second MOF with a different chemical system validates the prospective rational design of MOFs," Prof. Eddaoudi says. "Here we have shown that the rational design of MOFs is indeed a reality, thanks to the SBB approach."
The research team also examined the catalytic activity of gea-MOF-1 and found that it is catalytically active and has great potential for hydrocarbon separation. "We found that it can serve as an excellent recoverable catalyst for the solvent-free synthesis of carbonates under mild conditions," explains Dr. Valerio D'Elia, Research Scientist from the KAUST Catalysis Center (KCC) and a co-author of the paper.
"Our work also revealed that gea-MOF-1 can be employed as a C3H8/CH4 and n-C4H10/CH4 separation agent for natural gas upgrading because of its high affinity for C3H8 and n-C4H10 versus CH4 and CO2," Dr. Youssef Belmabkhout, co-author of the paper and Research Scientist in the FMD3 group, notes.
The researchers state that finding new nets that can be used as blueprints is becoming increasingly rare today, as many nets have already been predicted. "Our novel (3,18)-connected gea net has a great potential to be systematically targeted through the MBB or SBB approach for the synthesis of made-to-order MOFs for specific applications," says Dr. Guillerm.
Prof. Eddaoudi hopes that through the team's research work, others will realize the use of geometry and topology is often underestimated in solid-state materials design. "Use of this is critical if one wants to achieve made-to-order materials for specific applications," he says. In addition, he notes that the group's work "also highlights the great potential of MOFs for hydrocarbon separation, a feature which has barely been explored so far."
Related Links:
- by Caitlin Clark, KAUST News